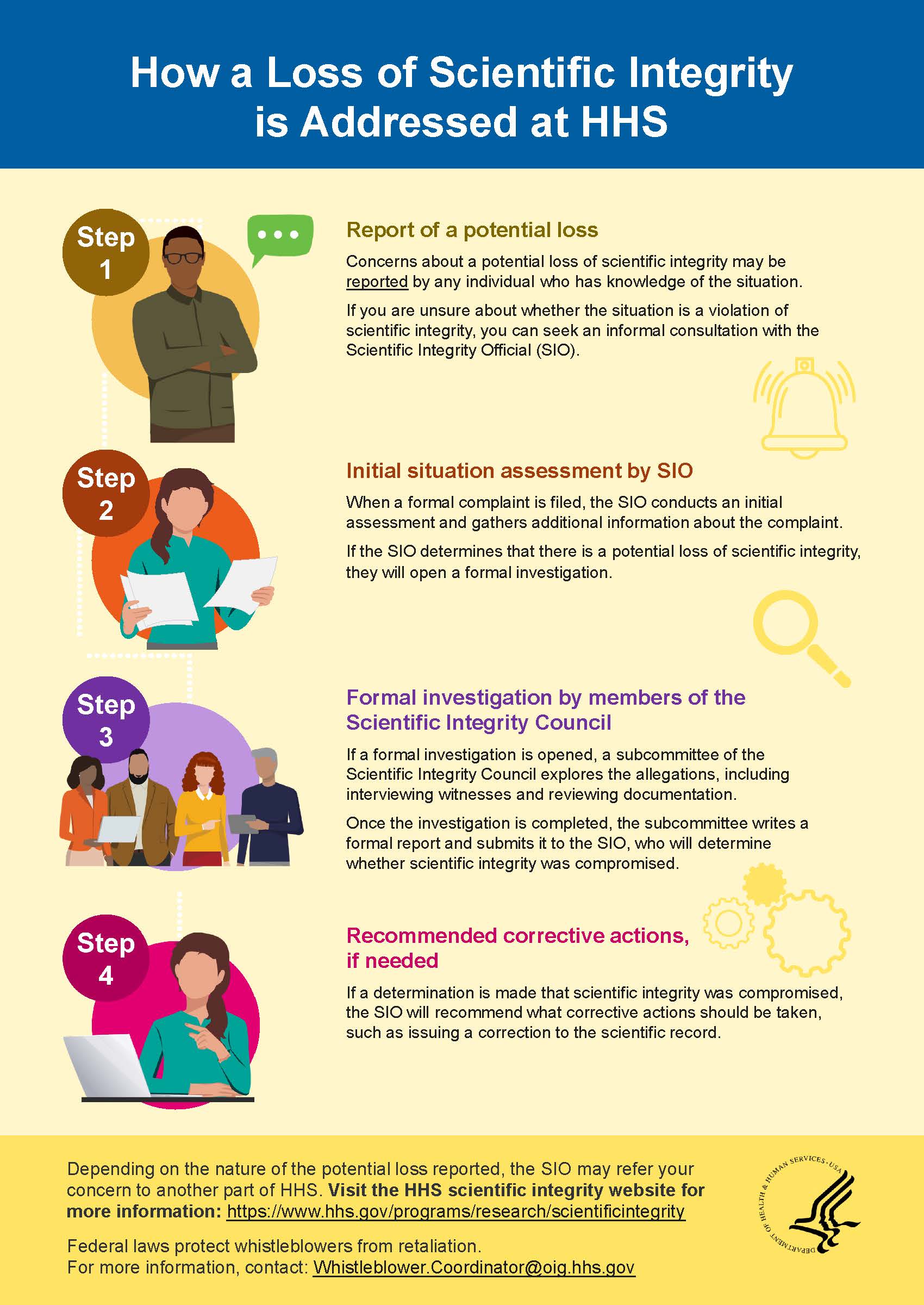
If you are unsure of whether a situation involves a potential loss of scientific integrity, if you would like to discuss how to prevent a loss of scientific integrity, or if you would simply like more information on the process before you submit a formal complaint, you are encouraged to schedule an informal consultation with the appropriate Scientific Integrity Official (SIO). Employees at CDC and FDA are encouraged to engage with the appropriate SIO listed below. All other employees are encouraged to direct questions or concerns, related to scientific integrity, to the HHS SIO.
The effective date of the HHS Scientific Integrity Policy is October 16, 2024. Only potential losses of scientific integrity that occur on or after the effective date of the HHS Scientific Integrity Policy can be addressed under the HHS Scientific Integrity Policy.
Who can I contact to submit a formal complaint about a potential loss of scientific integrity?
If you are aware of a potential loss of scientific integrity that has occurred at CDC or FDA, you are encouraged to report it to the relevant SIO. If you are aware of a potential loss of scientific integrity that has occurred anywhere else within HHS, please report it to the HHS SIO. If you are unsure where to report, you may report to the HHS SIO, who will direct your allegation as appropriate.
What information do I need to include in my formal complaint?
HHS can only address allegations related to a loss of scientific integrity that are credible and specific. We therefore encourage you to include as much detail as possible about what happened, how scientific integrity may have been compromised, when it happened, and who was involved. You do not need to have been directly involved or personally witnessed the incident to submit a complaint, but you should have sufficient knowledge of the incident to provide the SIO with details relevant to your allegation. The following guidance applies to complaints submitted to the HHS SIO.
- At a minimum, the complaint should include:
- A statement of facts. This should include a detailed description of what is alleged to have occurred, including, to the extent possible, dates, location, and how you became aware of the issue.
- The name(s) of the office(s) and/or individual(s) involved. Where possible, please include contact information for these offices or individuals.
- If possible, the complaint should include:
- Any evidence in support of your allegation, such as email records, electronic documents, or the storage location of any records or data.
- A statement indicating whether the allegation has been submitted elsewhere (e.g., EEO Office, Office of Research Integrity) and, if applicable, the outcome of that allegation. Previous submission elsewhere will not impact the assessment of your allegation.